
RIES
Research Institute for Electronic Science, Hokkaido University
北海道大学
電子科学研究所

LAST UPDATE 2022/07/13
-
研究者氏名
Researcher Nameテェーマイトリィ・ファーサイ Farsai TAEMAITREE
助教 Assistant Professor -
所属
Affiliation北海道大学 電子科学研究所
光科学研究部門 ナノ材料光計測研究分野
Research Institute for Electronic Science, Hokkaido University
Photonics and Optical Science, Laboratory of Nanomaterials and Nanoscopy -
研究キーワード
Research Keywordsナノ材料
薬輸送システム
表面修飾
ナノバイオサイエンス
Nanomaterials
Drug Delivery Systems
Surface Functionalization
Nano-bioscience
- 研究テーマ
Research Subject -
効率的ドラッグデリバリーシステム構築へ向けた薬剤ナノ粒子の細胞内動態の解明
Intracellular Investigation of Drug Nanoparticles toward Highly Efficient DDSs
研究の背景 Background of the Research
Since discovering the enhanced permeability and retention effect, nanoparticle-based drug delivery systems (DDSs) have gained much attention as a chemotherapeutic agent. Due to a significantly increased vascular permeability in tumor tissue and an underdeveloped lymphatic system, nanoparticles (NPs) can passively target and accumulate in tumor tissue without causing drug side effects. Nanocarrier-containing drug molecules (e.g., liposomes, polymer micelles, and dendrimers) have been explored extensively over the past decades. However, these nanocarriers are considered one of the main hindrances to drug delivery applications, possibly causing long-term side effects and increasing systematic toxicity. To overcome this limitation, the concept of alternative carrier-free DDSs composed exclusively of hydrophobic drugs or prodrugs was developed.
研究の目標 Research Objective
Despite the impressive therapeutic efficiency of carrier-free DDSs, a lack of knowledge and methodologies concerning the intracellular fate, degradation, and conversion into the active drug of NPs after internalization drastically hinders further developments toward clinical applications. Knowing (i) if NPs enter the cells; (ii) if they are decomposed before entering the plasma membrane or efficiently degraded inside the cells and converted into active drugs; and (iii) if the cellular enzyme level plays a role in these processes, is fundamental for evaluating the suitability of this innovative carrier-free DDSs towards further steps. Herein, we designed carrier-free DDSs for the study based on fluorescence microscopy to unravel the dynamics of NPs inside cancer cells, including internalization, intracellular localization, particle degradation, and therapeutic efficiency.
研究図Figures
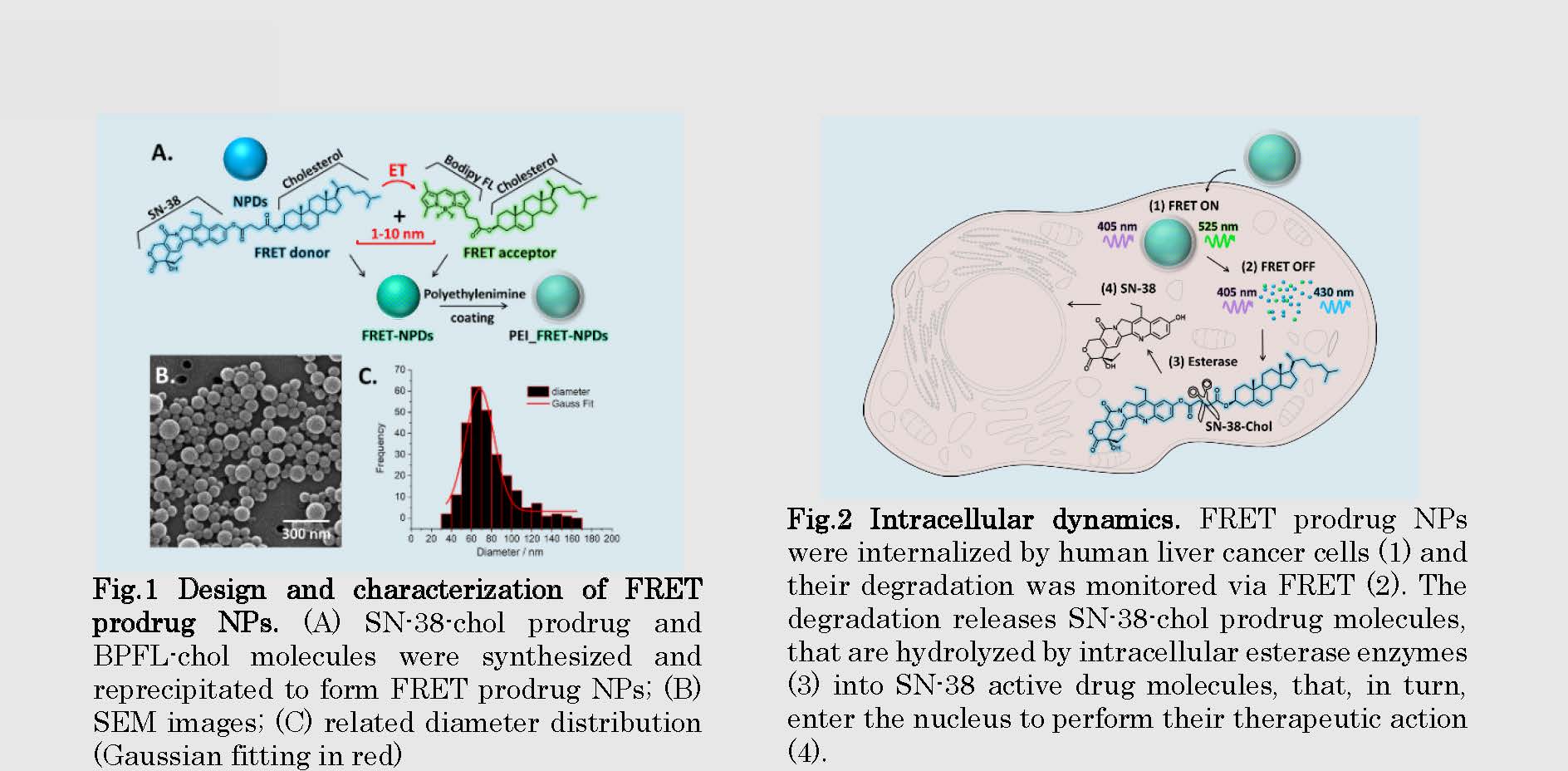
Fig.2 Intracellular dynamics. FRET prodrug NPs were internalized by human liver cancer cells (1) and their degradation was monitored via FRET (2). The degradation releases SN-38-chol prodrug molecules, that are hydrolyzed by intracellular esterase enzymes (3) into SN-38 active drug molecules, that, in turn, enter the nucleus to perform their therapeutic action (4).
論文発表 / Publications
Bull. Chem. Soc. Jpn., 92, 1305–1313 (2019)., Nanoscale, 12, 16710–16715 (2020)., Mol. Cryst. Liq. Cryst., 706, 116–121 (2020)., Chem. Lett., 50, 1555-1558 (2021)., J. Cryst. Growth, 572, 126265 (2021).
研究者連絡先 / HP
- farsai
 es.hokudai.ac.jp
es.hokudai.ac.jp - https://www.es.hokudai.ac.jp/labo/lnn/Top.html